Photochemical acti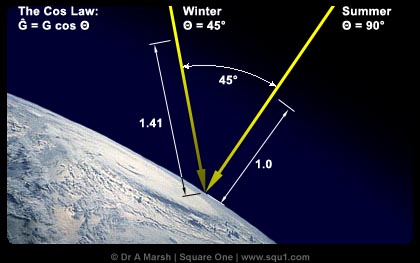 vity in the atmosphere increases during the spring at northern mid and high latitudes, partly due to an increase in solar incident radiation. The intensity of this radiation is largely due to the solar incidence angle, or the angle at which the sun's rays strike the Earth's surface. For instance, if the sun is directly overhead, the radiation is more intense, but if it is at an angle less than 90 degrees from the horizon, the radiation is spread out over a larger area and is not as intense. The sun is directly overhead during the equinoxes, which occur during fall and spring. At 90°N, in the Northern Hemisphere, the highest fluxes of solar insolation occur. From the autumnal
vity in the atmosphere increases during the spring at northern mid and high latitudes, partly due to an increase in solar incident radiation. The intensity of this radiation is largely due to the solar incidence angle, or the angle at which the sun's rays strike the Earth's surface. For instance, if the sun is directly overhead, the radiation is more intense, but if it is at an angle less than 90 degrees from the horizon, the radiation is spread out over a larger area and is not as intense. The sun is directly overhead during the equinoxes, which occur during fall and spring. At 90°N, in the Northern Hemisphere, the highest fluxes of solar insolation occur. From the autumnal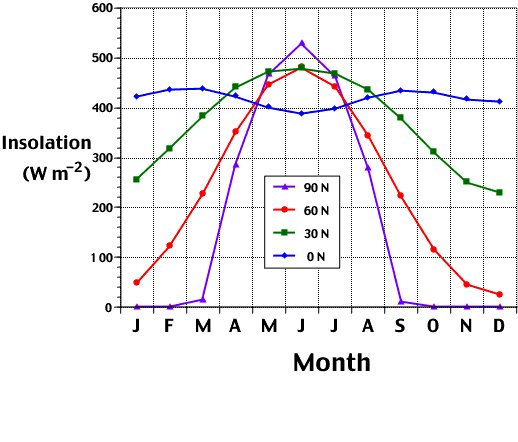 equinox (September 22) to the vernal equinox (March 21), this region receives no insolation, because the Earth's axis is tilted away from the sun. This makes the
equinox (September 22) to the vernal equinox (March 21), this region receives no insolation, because the Earth's axis is tilted away from the sun. This makes the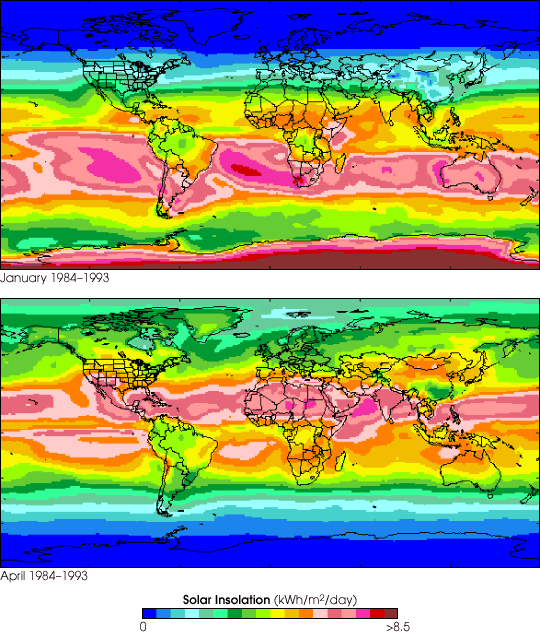 days shorter, until there is a period darkness. After March 21, there is a positive increase in solar insolation in this region, as the Earth's axis is shifted towards the sun, and day length increases until there is a period when the sun never sets. Therefore, solar insolation is more pronounced at mid and high latitudes than at the equator, making the chemistry in these regions very interesting to study as insolation levels increase in spring. For example, the rate of photolysis of O
days shorter, until there is a period darkness. After March 21, there is a positive increase in solar insolation in this region, as the Earth's axis is shifted towards the sun, and day length increases until there is a period when the sun never sets. Therefore, solar insolation is more pronounced at mid and high latitudes than at the equator, making the chemistry in these regions very interesting to study as insolation levels increase in spring. For example, the rate of photolysis of O3 increases during the spring, and hence does the reaction of O(1D) and H2O since:
O3 + hv -> O2 + O(1D)
O(1D) + H20 -> 2OH
In these coupled reactions, O3 is being split by the sun's energy into O2 and metastable oxygen, and then metastable oxygen reacts with water to yield two OH radicals.
In response to human forcing on the environment, the atmosphere tries to fix itself and come back to equilibrium. Increased radiation affects the atmosphere's oxidative processes, which are important in lessening human effects on the environment. These oxidative processes are driven by the sun's energy, and involve, but are not limited to, HOx(OH+HO2 ), O3 , NOx (NO+NO2 ), CO, and hydrocarbons. To st udy these processes, data was taken during the TOPSE (Tropospheric Ozone Production about the Spring Equinox) experiments, whose main objective was to investigate the tropospheric chemistry of mid to high latitudes in North America during the transitional period from winter to spring. These experiments were performed at 0-8 km, from February to May 2000. The mid latitudes measured were 40°-60°N and the high latitudes were 60°-8
udy these processes, data was taken during the TOPSE (Tropospheric Ozone Production about the Spring Equinox) experiments, whose main objective was to investigate the tropospheric chemistry of mid to high latitudes in North America during the transitional period from winter to spring. These experiments were performed at 0-8 km, from February to May 2000. The mid latitudes measured were 40°-60°N and the high latitudes were 60°-8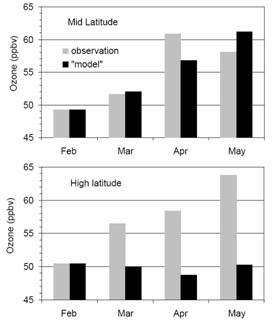 0°N.
0°N.
A box model was employed in the analysis of O3 and HOx photochemistry, which is the simplest model that can be used to study the atmosphere. In our work, we focused on the effects of chemical production and loss during TOPSE. The graph to the left compares model concentrations of ozone to observed concentrations of ozone in mid and high latitudes, to give some idea of how well the model was able to simulate the processes. When model and observation data disagree, it could be an indication that more processes are involved than were considered in the model. For further information, follow this link to view the abstract of the paper. For the full-length paper,
click here.
Website composed by Cindy Young.
 vity in the atmosphere increases during the spring at northern mid and high latitudes, partly due to an increase in solar incident radiation. The intensity of this radiation is largely due to the solar incidence angle, or the angle at which the sun's rays strike the Earth's surface. For instance, if the sun is directly overhead, the radiation is more intense, but if it is at an angle less than 90 degrees from the horizon, the radiation is spread out over a larger area and is not as intense. The sun is directly overhead during the equinoxes, which occur during fall and spring. At 90°N, in the Northern Hemisphere, the highest fluxes of solar insolation occur. From the autumnal
vity in the atmosphere increases during the spring at northern mid and high latitudes, partly due to an increase in solar incident radiation. The intensity of this radiation is largely due to the solar incidence angle, or the angle at which the sun's rays strike the Earth's surface. For instance, if the sun is directly overhead, the radiation is more intense, but if it is at an angle less than 90 degrees from the horizon, the radiation is spread out over a larger area and is not as intense. The sun is directly overhead during the equinoxes, which occur during fall and spring. At 90°N, in the Northern Hemisphere, the highest fluxes of solar insolation occur. From the autumnal equinox (September 22) to the vernal equinox (March 21), this region receives no insolation, because the Earth's axis is tilted away from the sun. This makes the
equinox (September 22) to the vernal equinox (March 21), this region receives no insolation, because the Earth's axis is tilted away from the sun. This makes the days shorter, until there is a period darkness. After March 21, there is a positive increase in solar insolation in this region, as the Earth's axis is shifted towards the sun, and day length increases until there is a period when the sun never sets. Therefore, solar insolation is more pronounced at mid and high latitudes than at the equator, making the chemistry in these regions very interesting to study as insolation levels increase in spring. For example, the rate of photolysis of O
days shorter, until there is a period darkness. After March 21, there is a positive increase in solar insolation in this region, as the Earth's axis is shifted towards the sun, and day length increases until there is a period when the sun never sets. Therefore, solar insolation is more pronounced at mid and high latitudes than at the equator, making the chemistry in these regions very interesting to study as insolation levels increase in spring. For example, the rate of photolysis of O
