 Factors Controlling tropospheric O
Factors Controlling tropospheric O3 , OH, NOx , and SO2 over the Tropical Pacific during PEM-Tropics B
 Factors Controlling tropospheric O
Factors Controlling tropospheric O3 , OH, NOx , and SO2 over the Tropical Pacific during PEM-Tropics B
The Pacific Exploratory Mission (PEM)-Tropics B in March-April of 1999, surveyed Hawaii , Fiji , Tahiti , and Easter Island from the boundary layer up to 12km, and sampled the tropical lower troposphere below 6km mostly between Hawaii and Tahiti . Observations from PEM-Tropics B were used to examine factors that govern the chemistry of O3, OH, NOx, and SO2 over the tropical Pacific. Let's have a closer look at each of these chemical species.
Tropospheric ozone is either made photochemically in the troposphere, or is transported from the stratosphere. Ozone is created photochemically through a series of complex reactions between ultraviolet light, and hy
drocarbons and nitrogen oxides, which come from vehicle emissions and the combustion of other fossil fuels. Stratospheric ozone is produced when ultraviolet light strikes an O2 molecule, splitting it into two oxygen atoms. When three single oxygen atoms collide, ozone is created. This ozone can be transported into the troposphere by way of upper layer trough and lower layer cut-off activities, as well as by Hadley circulation and Brewer Dobson circulation.
The destruction of ozone is necessary for the creation of OH, as can be seen in the following chemical reactions:
O3 + hv -> O2 + O(1D)
O(1D) + H20 -> 2OH
OH disposes of many of the nasty pollutants that get trapped in the atmosphere. With an unpaired electron in its outermost orbital, it readily combines with and oxidizes compounds such as hydrocarbons, hydrogen sulfide, and carbon monoxide, which are poisonous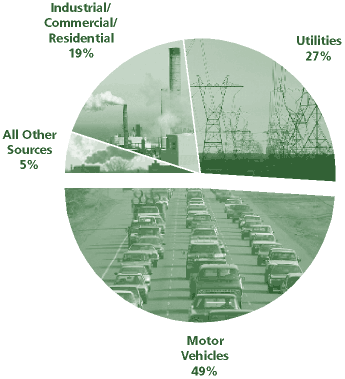 .
.
NOx, or the nitrogen oxides, are a group of very reactive gases that are all composed of differing amounts of nitrogen and oxygen. Nitrogen oxides form when fuel is burned at high temperatures, the primary sources being motor vehicles, electric utilities, and other industrial, commercial, and residential sources that burn fuels. However, a smaller amount of NOx is also produced naturally through lightning. Most of these gases are colorless and odorless, but there are exceptions. For example NO2, or nitrogen dioxide, is made evident by the reddish-brown
color that hangs over ma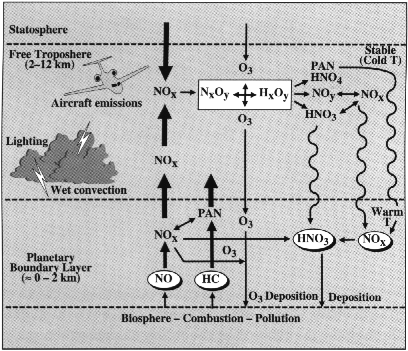 ny cities. Some other derivatives of NOx are nitric acid, nitrous oxide (a greenhouse gas), nitrates, and nitric oxide. NOx, along with volatile organic compounds (VOCs), react in the presence of heat and sunlight to form ground-level ozone or photochemical smog. Sulfur dioxide and NOx react with other substances to form acid rain, and NOx also reacts with organic compounds to form toxins, like the nitrate radical, nitroarenes, and nitrosamines, which may cause mutations.
ny cities. Some other derivatives of NOx are nitric acid, nitrous oxide (a greenhouse gas), nitrates, and nitric oxide. NOx, along with volatile organic compounds (VOCs), react in the presence of heat and sunlight to form ground-level ozone or photochemical smog. Sulfur dioxide and NOx react with other substances to form acid rain, and NOx also reacts with organic compounds to form toxins, like the nitrate radical, nitroarenes, and nitrosamines, which may cause mutations.
SO2 in the atmosphere is created from dimethyl sulfide (DMS), which is produced from phytoplankton in the ocean. 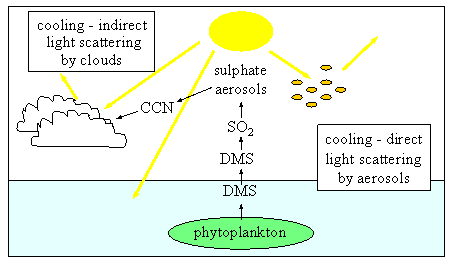 SO
SO2 then produces sulfate aerosols, which are tiny liquid and solid particles. The concentrations of aerosols are increasing with time. Aerosols can serve as effective cloud condensation nuclei, so if concentrations of aerosols increase, more of the Earth will be covered by clouds. Clouds have a high albedo, or reflectivity, meaning a significant amount of sunlight is reflected off clouds and back into space. If there are more clouds, this can cause a cooling effect. On the other hand, aerosols can absorb long-wave radiation, which can cause a warming effect. However, the net effect of aerosols on climate is thought to be one of cooling.
Another source o f aerosol particles is methyl iodide. Like DMS, it is released by phytoplankton, although seaweed provides a much larger source. In the atmosphere, it is transformed into other forms of iodine, which are associated with the smallest aerosol particles in the atmosphere. The mechanisms of producing methyl iodide are different than those for DMS and are little understood. However, it is known that the methyl iodide released from seaweed is a stress response.
f aerosol particles is methyl iodide. Like DMS, it is released by phytoplankton, although seaweed provides a much larger source. In the atmosphere, it is transformed into other forms of iodine, which are associated with the smallest aerosol particles in the atmosphere. The mechanisms of producing methyl iodide are different than those for DMS and are little understood. However, it is known that the methyl iodide released from seaweed is a stress response.
PEM-Tropics A was a similar experiment, only it was performed in September-October of 1996.
When PEM-Tropics experiments A and B were compared, it was evident that pollutants were being transported from different regions of origin, and therefore were having opposite effects on the oxidizing capacity of the atmosphere, or OH concentration, over the tropical Pacific. Experiment A was influenced greatly by biomass burning plumes.
 Biomass burning is the burning of the world's living and dead vegetation for land clearing, land use change, and natural burning resulting from lightning-induced fires. It is believed that the vast majority of these fires are caused by humans, and they are a significant source of global emissions. Combustion products of biomass burning include carbon dioxide, carbon monoxide, methane, nonmethane hydrocarbons, ni tric oxide, nitrous oxide, and atmospheric particulates. It has been found that biomass burning may release significant amounts of methyl bromine, which can destroy stratospheric ozone. With more biomass consumed by fire than anywhere else on Ear
Biomass burning is the burning of the world's living and dead vegetation for land clearing, land use change, and natural burning resulting from lightning-induced fires. It is believed that the vast majority of these fires are caused by humans, and they are a significant source of global emissions. Combustion products of biomass burning include carbon dioxide, carbon monoxide, methane, nonmethane hydrocarbons, ni tric oxide, nitrous oxide, and atmospheric particulates. It has been found that biomass burning may release significant amounts of methyl bromine, which can destroy stratospheric ozone. With more biomass consumed by fire than anywhere else on Ear
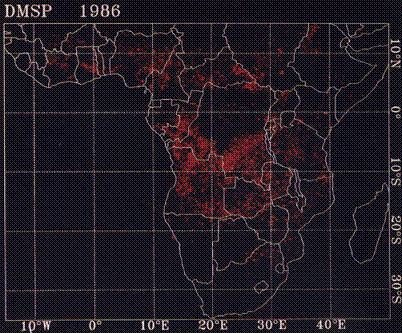 th, the savanna grasslands of Africa of been labeled the “burn center” of the planet.
th, the savanna grasslands of Africa of been labeled the “burn center” of the planet.
The sources of pollutants in PEM-Tropics B were from northern industrialized continents. Faster convective transport and less influence from long-range transport of pollutants in PEM-Tropics B makes it useful to use in studying effects of convection chemistry over the tropics. For more general information, view the abstract of the paper by clicking here. To view the entire paper, use this link.
webpage composed by: Cindy Young